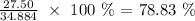Answer:
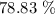
Step-by-step explanation:
Here, we want to calculate the percentage yield of the reaction
Mathematically, we have that as:
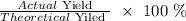
The mass of the product NO given in the question is the actual yield which is 27.50 g
Now, let us get the theoretical yield
We find the number of moles of nitrogen gas that reacted
Mathematically, that is the mass of nitrogen gas divided by the molar mass
The molar mass of the nitrogen gas is 28 g/mol
The number of moles is thus:
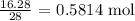
From the equation of reaction, 1 mole of N2 yields 2 moles of NO
Thus, 0.5814 mol of N2 will yield:
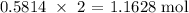
Now, to get the mass of NO produced, we multiply this number of moles by the molar mass of NO
The molar mass of NO is 30 g/mol
The mass that was produced is thus:
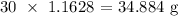
Finally, we have the percentage yield as: