Answer:
10.81amu
Explanations:
In order to get the average atomic mass of an element, we need the following parameters:
• Natural Abundance (NA),: The percentage of atoms for an element that is a specific isotope.
• Mass (m) ,of each isotope
For the given element (Boron-10 and Boron-11), the natural abundances are 19.78% and 80.22% respectively.
The atomic masses of Boron-10 and Boron-11 are 10.0129amu and 11.0093amu respectively
The formula for calculating the average atomic mass of the element is expressed as:
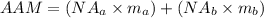
Substitute the given parameters into the formula to have:
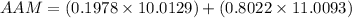
Simplify the resulting expression to have:
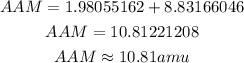
Therefore the average atomic mass of Boron is 10.81amu to two decimal places.