Answer:
Percent yield = 27.3%.
Step-by-step explanation:
First, let's write the chemical equation:
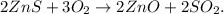
The limiting reactant, in this case, would be O2 because we have an excess of ZnS. So, we have to convert 42.5 g of O2 to moles. Remember that the molar mass of O2 is 32 g/mol (you can calculate the molar mass of a compound using the periodic table). The conversion will be:
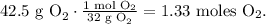
With this value, we're going to find the number of moles of SO2 produced by 1.33 moles of O2. You can see in the chemical equation that 3 moles of O2 reacted produces 2 moles of SO2, so the calculation would look like this:
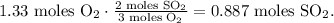
The next step is to find the mass of SO2 based on its number of moles and the molar mass of SO2 which is 64 g/mol, like this:
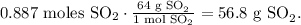
And finally, we replace the values that we have in the formula of percent yield:
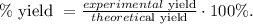
Our experimental yield is the mass that we obtained of SO2 which is 15.5 g and the theoretical yield is the mass that we found through stoichiometry which is 56.8g:
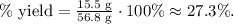
The percent yield of this reaction would be 27.3%