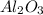1) Write the proper formula for Mg2+ and Br-
Balance the charges.
Mg2+: Multiply by 1. This gives a total of two positive charges.
Br-: Multiply by 2. This gives a total of two negative charges.
Use multipliers as subscripts
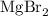
2) Write the proper formula for Al3+ and O2-
Al3+: Multiply by 2. This gives a total of six positive charges.
O2-: Multiply by 3. This gives a total of six negative charges.
Use multipliers as subscripts