Answer: 189.5g of Ag would be produced from 0.88 moles of Ag2S
Step-by-step explanation:
The question requires us the amount of Ag, in grams, that would be produced from 0.88 moles of Ag2S, according to the following equation:
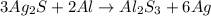
To solve this problem, which is a mol to mass stoichiometry problem, we'll need to follow the steps:
moles of Ag2S → moles of Ag → mass of Ag
To do that, we'll need to use the molar mass of Ag (107.87 g/mol) and the mole ratio between Ag2S and Ag, as given by the balanced chemical equation (6 moles of Ag are produced from 3 moles of Ag2S).
We can calculate the number of moles of Ag that would be produced from 0.88 moles of Ag2S as:
3 mol Ag2S -------------------------- 6 mol Ag
0.88 mol Ag2S --------------------- x
Solving for x, we'll have:
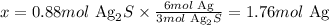
Therefore, 1.76 moles of Ag would be produced from 0.88 moles of Ag2S.
Next, we need to convert the number of moles of Ag calculated to its correspondent mass. Knowing that the molar mass of Ag is 107.87 g/mol, we can calculate:
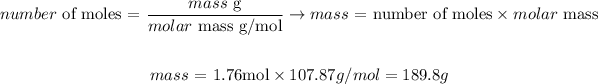
Therefore, 189.5g of Ag would be produced from 0.88 moles of Ag2S.