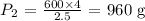Answer:
Step-by-step explanation:
Here, we want to calculate the pressure used at the new volume
It is expected that at a higher pressure, the volume will decrease
Thus, the weight and the pressure are inversely proportional
Mathematically:
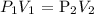
The above is according to Boyle's law which states that volume and pressure are inversely proportional
where:
V1 is the initial volume which is 4 cc
P1 is the initial weight which is 600 g
V2 is the final volume which is 2.5 cc
P2 is the final pressure which is unknown
Substituting the values: