Answer:
0.124 M.
Step-by-step explanation:
What is given?
Volume 1 (V1) = 239 mL,
Concentration 1 (C1) = 0.274 M,
Volume 2 (V2) = 239 mL + 288 mL = 527 mL.
What do we need? Concentration 2 (C2).
Step-by-step solution:
To solve this problem, we have to use the following formula:

Where the initial concentration (C) and volume (V) are represented with subindex 1, and the final concentration and volume are represented with subindex 2.
The problem is telling us that we are adding 288 mL of water to 239 mL, so the final volume is the sum of these two volumes, i.e., 527 mL.
As we want to find the final concentration, C2, we just have to solve for this unknown value and replace the values that we have, like this:
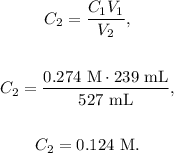
The answer would be that the final concentration of the solution is 0.124 M.