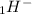We are asked to write shortened notation for the ion symbol next to each element given below.
Convention:
First of all, write the shortened notation of the element.
The superscript (at the top) is the number of charges on the ion and also a + sign for a positive ion and - sign for a negative ion.
When an atom loses an electron then the charge is positive (+).
When an atom gains an electron then the charge is negative (-)
A.) Calcium loses 2 negative electrons
Since it loses 2 electrons it will have 2+ superscript.
Calcium has an atomic number of 20 (the number of protons)
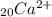
B.) Chlorine gains 1 negative electron
Since it gains 1 electron it will have - superscript (we don't write 1 when the charge is 1)
Chlorine has an atomic number of 17 (the number of protons)
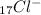
C.) Hydrogen gains 1 negative electron
Since it gains 1 electron it will have - superscript
Hydrogen has an atomic number of 1 (the number of protons)