1) Molar mass of Allicin.
1.1- Write the formula.
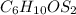
1.2- Look for the molar mass of each element in the formula.
C: 12.011 g/mol
H: 1.008 g/mol
O: 15.999 g/mol
S: 32.06 g/mol
1.3- Count how many atoms are there in the formula.
C: 6
H: 10
O: 1
S: 2
1.4- Set the equation.
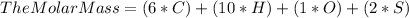
Plug in the known values.
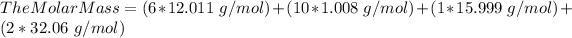
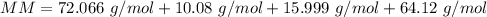
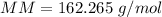
The molar mass (MM) of Allicin (C6H10OS2) is 162.265 g/mol.
2) Moles of Allicin in the sample.
Allicin sample: 4.20 mg
The molar mass of Allicin (C6H10OS2) is 162.265 g/mol.
2.1- Convert mg to g.
1g = 1000 mg
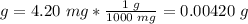
2.2- Convert grams to moles.
The molar mass of Allicin (C6H10OS2) is 162.265 g/mol.
Allicin sample: 0.00420 g
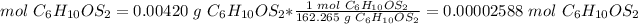
There are 0.00002588 moles of Allicin (C6H10OS2) in 4.20 mg of substance.
The result can also be expressed as 2.588*10^(-5) mol.
3) Molecules of Allicin in the sample.
The Avogadro's number is 6.022*10^(23).
1 mol C6H10OS2 = 6.022*10^(23) molecules C6H10OS2
Allicin sample: 0.00002588 mol.
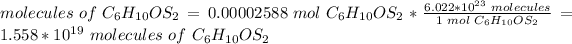
There are 1.558*10^(19) molecules in the sample.
4) C atoms in the sample of Allicin.
The ratio of carbon in one molecule of Allicin is 6 C atoms: 1 molecule of Allicin.
Allicin sample: 1.558*10^(19) molecules C6H10OS2.
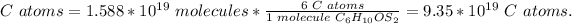
There are 9.35*10^(19) C atoms in the sample.