Answer:
Total heat removed = 473.04 kCal
Step-by-step explanation:
Heat removed to convert the 116°C to 100°C steam
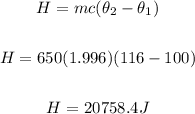
Heat removed from 100°C of steam to 100°C of water (Latent heat of condensation)
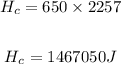
Heat removed from 100°C water to 0°C water
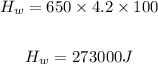
Heat removed from 0°C water to 0°C ice
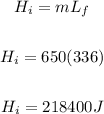
Total heat removed = 20758.4J + 1467050 + 273000 + 218400
Total heat removed = 1979208.4 J
Convert to kilocalorie
Total heat removed = 1979208.4/4184
Total heat removed = 473.04 kCal