Answer:
D. 32.
Step-by-step explanation:
Remember the formula of atomic mass:
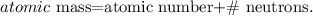
If you go to see the periodic table, you can note that the atomic mass of cobalt is 58.9 amu rounded to 59 amu. Let's solve for '# neutrons' and replace the atomic mass (59) and the atomic number (27) (remember that the number of protons is the same that the atomic number), like this:
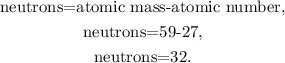
The answer would be that the number of neutrons to make a cobalt atom most stable is D. 32.