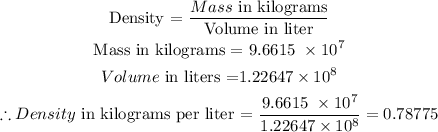Answer
The density of the oil in kilograms per liter is 0.78775
Step-by-step explanation
Note: 1 gallon = 3.78541 liters
1 pound = 0.453592 kilogram
Given:
The gallons (volume) of crude oil = 3.24 x 10⁷ gallons
The mass of the crude oil = 2.13 x 10⁸ pounds
Conversion:
The volume in liters of 3.24 x 10⁷ gallons of crude oil will be
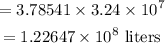
Also, the mass in kilograms of 2.13 x 10⁸ pounds of crude oil will be
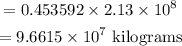
Now, we shall use the formula below to calculate the density