Given that;
The blueprint shows an apartment with an area of 15 square inches.
With scale
1 inch : 8 feet
Recall that;
Area of a square is;
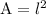
Let l represent the length of the side on the blueprint;
The actual length will be;
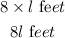
So, the actual Area will be;
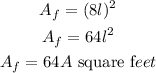
substituting the valuye of the blueprint Area;