ANSWER:
1000 L
Explanation:
The first thing is to convert the unit of both pressures to atm, with the help of the conversion equivalences shown, just like this:

Now, applying Boyle's Law, we calculate the value of the volume, like this:
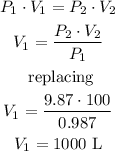
The volume of gas is 1000 L