Temperature is a way to measure the kinetic energy of the molecules of gases. The kinetic energy is a kind of energy that a moving body has. it is related to the mass of the body and the speed they are moving. The kinetic energy can be calculated as:
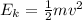
Where Ek is the kinetic energy, m is mass of the molecules and v is the speed of the molecule
Knowing that gasses with the same temperature have same kinetic energy, then gasses with larger mass will move slower and viceversa.
Since the molar mass of those gases are:
He: 4.00 g/mol
SF6: 146.06 g/mol
SO2: 64.066 g/mol and
Ar: 39.948 g/mol
the ligther gasses move faster and the hevier slower, so we just have to order them by increasing mass:
He, Ar, SO2 and SF6