ANSWER
Definition of an acid; is a substance that produced hydrogen ions (H+) as the only positive ion when dissolved in water or in an aqueous solution. it increases the concentration of hydrogen ions in an aqueous solution.
Definition of the base; are hydroxide compounds that give hydroxide ion (OH-) on dissociation in water. it increases the concentration of hydroxide ions in an aqueous solution.
Why do we use the definition: it helps us to understand the acid-base properties of substances in an aqueous medium. neutralization, hydrolysis, and strength of acids and bases
Step-by-step explanation:
Arrhenius defined acid and base in the following way
According to Arrhenius, he defined an acid as a substance that produced hydrogen ions (H+) as the only positive ion when dissolved in water or in an aqueous solution. it increases the concentration of hydrogen ions in an aqueous solution.
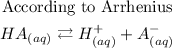
Bases are hydroxide compounds that give hydroxide ion (OH-) on dissociation in water. it increases the concentration of hydroxide ions in an aqueous solution.
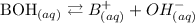
Arrhenius's definition of acid and base is still in use because it helps us to understand the acid-base properties of substances in an aqueous medium. neutralization, hydrolysis, and strength of acids and bases