ANSWER
3.7128 x 10⁻³⁴ J
Step-by-step explanation
The energy carried in a one-quantum change is the product of Planck's constant, h, and the frequency of vibration, f,
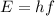
Planck's constant is 6.63 x 10⁻³⁴ J*s and, in this case, the frequency of vibration is 0.56 Hz. So, the energy carried away is,
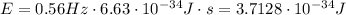
Hence, the energy carried away in a one-quantum change is 3.7128 x 10⁻³⁴ J.