We are asked to determine the density of a gulf ball given its mass and volume. To do that, we will use the formula for density:
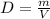
Where:
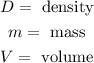
To determine the volume we will use the fact that the gulf ball can be approximated to a sphere and the volume of a sphere is given by:
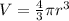
Where:
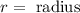
We are given the diameter. We know that the diameter is twice the radius, therefore:
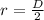
Substituting the value of the diameter we get:
![r=\frac{4.287\operatorname{cm}}{2}]()
Solving the operations:
![r=2.144\operatorname{cm}]()
Now, we substitute the value of the radius in the formula of the volume:
![V=(4)/(3)\pi(2.144\operatorname{cm})^3]()
Solving the operation we get:
![V=41.282\operatorname{cm}^3]()
Now, we substitute the value of the volume and the mass in the formula for density:
![D=\frac{45.87g}{41.282\operatorname{cm}^3}]()
Solving the operation:
![D=1.11\frac{g}{\operatorname{cm}^3}]()
Therefore, the density of the ball is 1.11 g/cm^3.