Answer:
2 oxygen ions
Step-by-step explanation
Oxygen is a non-metal with an atomic number of 8. The electronic configuraton of the element is given as:
![O;\text{ }1s^22s^22p^^4]()
If a an unknown metal with a charge of 4 combines with oxygen, the required reaction will be:
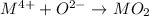
Since oxygen is in group VI of the periodic table and has a oxidation number of -2, to form a neutral compound with charge of +4, this means that 2 oxygen ion will combine with the metal to form a neural compound MO2