First, we need the reaction to start the exercise.
HCOOH(aq) + H2O(l) = H3O+(aq) + HCOO-(aq)
HCOOK => HCOO- + H+
------------------------------------------------------------------------
pH)
We have here an ionic equilibrium, HCOOH is a weak acid.
pKa = 3.77 (from tables)
The concentration of HCOOH 0.3 M
The concentration of HCOO- = 0.52 M HCOOK.
If we propose Ka for HCOOH(aq)
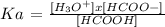
We clear (H3O+) and we apply -log and we get this:
![pH\text{ = }pKa+log(([HCOO^-])/([HCOOH]))](https://img.qammunity.org/2023/formulas/chemistry/college/n5g03lxw3rk86p7i7ekusgpj10pyygj5ml.png)
![pH=3.77+log(([0.52])/([0.30]))\text{ }](https://img.qammunity.org/2023/formulas/chemistry/college/njrrp0qebxk3afrstekroji3cxxsp60uce.png)
Answer: pH = 4.01
--------------------------------------------------------------------------------------------------
pOH)
pH + pOH = 14
(This formula appears from the ionic equilibrium of water)
pOH = 14 - 4.01 = 9.99
Answer: pOH= 9.99
--------------------------------------------------------------------------------------------------