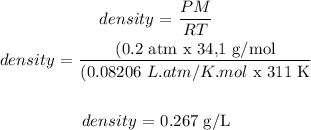Answer
Density = 0.267 g/L
Step-by-step explanation
Given:
Pressure of H2S = 0.2 atm
Temperature = 311 K
We know:
The molar mass of H2S = 34,1 g/mol
R constant = 0.08206 L.atm/K.mol
Solution:
From the ideal gas law:
PV = nRT
We know that:
density = m/V
n = m/M
Therefore we can use the following equation to solve for density of H2S