The first thing will be to finish the reaction that happens in the experiment to the reactional HCl and sodium bicarbonate (NaHCO3). The balanced reaction is the following:
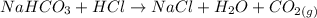
Now, we must determine which is the limiting reactant. To do this we are going to convert all the data they give us to moles.
For HCl we are given the molarity, so the moles will be:
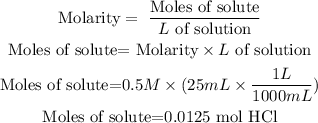
Now the moles of sodium bicarbonate are found using its molar mass:
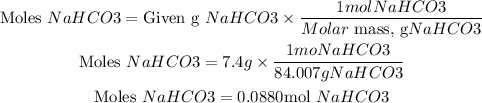
By stoichiometry, we have that one mole of NaHCO3 reacts with one mole of HCl. We have more moles of NaHCO3 than HCl. Therefore, HCl will be the limiting reactant.
So the reaction will occur according to the number of moles of HCl. Now the ratio between HCl and CO2 gas formed is 1 to 1. For one mole of HCl that reacts, 1 mole of CO2 will be formed.
so, the number