EXPLANATION
The equation for half-life is given by the following formula:
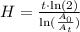
Replacing terms:
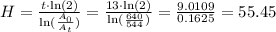
The half-life time is H =55.4 hours.
B) After three days, that is, 72 hours, the amount of substance will be given by the following relationship:
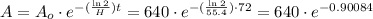
Multiplying terms:
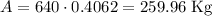
There will be 259.96 Kg after 3 days.
C) In order to compute the number of days that will take to the substance to reach a concentration equal to 185 Kg, we need to apply the following formula:
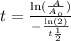
Replacing terms:
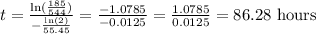
It will take 86.28 hours to the substance to reach 185 Kg.