To solve this problem, we must take into account Avogadro's number. Avogadro's number tells us that in one mole of any substance there are 6.022x10^23 atoms. Applying this relationship we have that the moles of potassium (K) are:
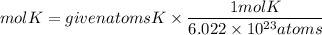
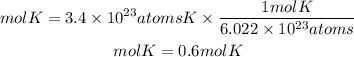
Now, to go from moles to grams, we must multiply the moles by the molar mass of potassium. The molar mass of potassium is 39.1g/mol. So, the grams of potassium will be:
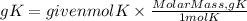
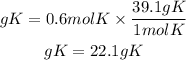
Answer: In 3.4x10^23 atoms of potassium there are 22.1 grams