Firstly we will determine the average or the mean of the objects density:
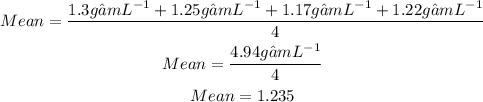
Now we will calculate the deviation. The deviation is how much is trial is different from the average. We take the absolute value so the answers can be positive:

We will determine the average of the deviation:
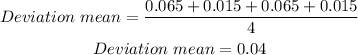
To determine the percent deviation we:

Answer: The percent deviation from the mean is 3.24%,