Given
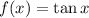
To fill the blanks.
Now,
The grpah of f(x)=tanx is given as,
Therefore, from the graph,
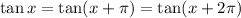
Then,
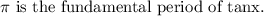
Also,
For x=0, tan(0)=0.
Therefore, the y-intercept is (0,0).
The points for the function f(x)=tanx is,
![\begin{gathered} x=(\pi)/(3),\text{ y=tan(}(\pi)/(3))=\sqrt[]{3} \\ x=(\pi)/(4),y=\tan (\pi)/(4)=1 \end{gathered}](https://img.qammunity.org/2023/formulas/mathematics/high-school/9hes9bxsk64n4i8d68jspwplps9npksylj.png)
Since
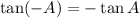
Then, the points of the graph y=tanx is,
![(-(\pi)/(3),-\sqrt[]{3}),(-(\pi)/(4),-1),(0,0),((\pi)/(3),\sqrt[]{3}),((\pi)/(4),1)](https://img.qammunity.org/2023/formulas/mathematics/high-school/zs04mh15zfv49fyjeifiiazigi3n88wzwk.png)
Hence, the answer should be in the order,
i)
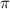
ii)
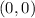
iii)
![(-(\pi)/(3),-\sqrt[]{3}),(-(\pi)/(4),-1),(0,0),((\pi)/(3),\sqrt[]{3}),((\pi)/(4),1)](https://img.qammunity.org/2023/formulas/mathematics/high-school/zs04mh15zfv49fyjeifiiazigi3n88wzwk.png)