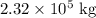The density is given by:
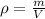
where V is the volume and m is the mass.
To determine the mass we have to solve the equation for m:
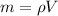
Now, before we can calculate the mass we have to convert the volume given to cubic meter, this comes from the fact that the density is given in g/cm^3 units. We have to remember that a ft is equal to 30.48 cm, then we have:
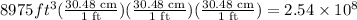
Hence the volume of the iceberg is:
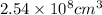
Now that we have the volume in the correct units we plug its value and the density in the equation for the mass above:
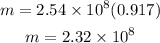
Hence the mass of the iceber is 2.32x10^8 g.
Therefore the mass of the iceberg in kilograms is: