Answer:
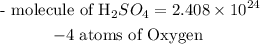
Explanations:
Given the following parameters
Moles of sulfuric acid = 14 moles
According to the Avogadro's constant;
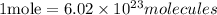
The number of molecules of 14 moles of sulfuric acid is calculated as:
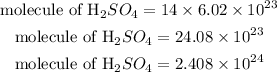
Hence the molecule of sulfuric acid that is contained in 14moles if sulfuric acid is 2.408 * 10^24 molecules
Since the chemical formula of sulfuric acid is expressed as H₂SO₄. This shows that the compound has 2 atoms of Hydrogen, 1 atom of sulfur, and 4 atoms of oxygen.
Hence the number of atoms of oxygen contained in this sample is 4 atoms