Isopropyl alcohol + water = Solution
Solute = Isopropyl alcohol
Solvent = water
% V/V = mL of solute / 100 ml of solution
Now we have 655 mL of solution.
32.0 % V/V means 32 mL of Isopropyl alcohol dissolved in 100 mL of solution.
So,
32 mL Isopropyl alcohol ------------------- 100 mL Solution
x ------------------- 655 mL Solution
x represents the volume of Isopropyl alcohol in 655 mL of solution
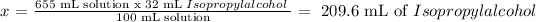
Now
Alcohol: 209.6 mL
Water: 655 mL (solution = Alcohol + Water) - 209.6 mL (alcohol) = 445.4 mL of water