3.786137931L of chlorine are needed.
1st) It is necessary to write the balanced equation of the reaction between chlorine gas (Cl2) and sodium (Na) to produce sodium chloride (NaCl):
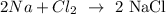
Now we know that 1 mol of chlorine gas is needed to react with 2 moles of sodium in the production of sodium chloride. With the molar mass of Na (23.0g/mol) and Cl2 (71.0g/mol), we can see that 71.0g of Cl2 are needed to react with 46.0g of sodium.
2nd) Knowing that 71.0g of Cl2 are needed to react with 46.0g of sodium, we can use a mathematical rule of three to calculate the amount of Cl2 that will react completely with 12.0g of sodium:
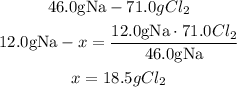
3rd) Now it is necessary to convert the mass of chlorine gas into moles, so we can use it in the Ideal gas formula:
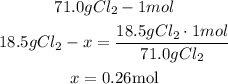
So, 0.26 moles of chlorine gas are needed.
4th) With the formula of Ideal gases and replacing the values of Pressure (P), number of moles (n) and Temperature (T, in Kelvin), we can calculate the volume of Cl2:
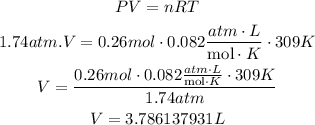
Finally, 3.786137931L of chlorine are needed to react completely with 12.0g of sodiu.