A net ionic equation is the one that only includes the ions that are involved in the formation of the solid.
According to the information the solid formed is iron (III) phosphate, and the ions involved are Fe3+ and PO43-.
It means that the net ionic equation is:
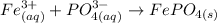
The correct answer is the second choice.