Here, there are 2 conditions of the gas here.
The next equation is used: (1)
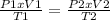
Data: conditions 1
P1 = pressure, in mmHg = 571.6 mmHg
T1 = temperature, in K = 25 °C + 273 = 298 K
V1 = 2,275 mL
-------------
Data: conditions 2
P2 = needs to be calculated
T2 = 175 °C + 273 = 448 K
V2 = 1,255 mL
--------------
From (1), P2 is cleared:
(P1 x V1/T1) x T2/V2 = P2
(571.6 mmHg x 2,275 mL/298 K) x (448 K/1,255 mL) = P2
1558 mmHg = P2
Answer: P2 = 1558 mmHg