ANSWER
Option C
Step-by-step explanation
Given that;

In the reaction above, we have the following data
At the reactants side;
3 atoms of manganese
4 atoms of nitrogen
1 atom of sodium
1 atom of fluorine
At the products side
1 atom of manganese
4 atoms of fluorine
3 atoms of sodium
1 atom of nitrogen
To balance the above equation, apply the law of conservation mass
Law of conservation of mass states that matter can neither be created nor destroyed but can e transformed from one formato another.
To balance the equation, 1 mole of Mn3N4 reacts with 12 moles of Na Tto give 3 moles of MnF4 and 4 moles of Na3N
So, the new equation becomes
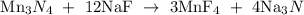
The following data can be deduced in the above equation
At the reactants side
3 atoms of Mn
4 atoms of N
12 atoms of Na
12 atoms of F
At the products side
3 atoms of Mn
12 atoms of F
12 atoms of Na
4 atoms of N
Looking atthe vabove data, the number of atoms of each element at the reactants side is equal to the number of atoms of same elements at the products side.
Hence, the correct answer is option Ce
u