Given
Volume of the oxygen gas is

The temperature is
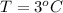
Pressure is
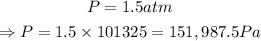
To find
The translational kinetic energy
Step-by-step explanation
The translational kinetic energy is given by
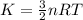
For ideal gas,

Thus,
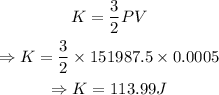
Conclusion
The translational kinetic energy is 113.99J