Exponential Decay
The model for the exponential decay of a quantity Mo is:
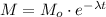
Where λ is a constant and t is the time.
The half-life of a radioactive isotope is the time it takes to halve its initial mass. It can be calculated by making M = Mo/2 and solving for t:
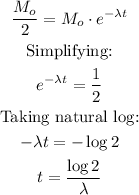
It's required to calculate the remaining mass of an isotope of Mo = 175 gr after 5 half-lives have passed, that is. we must calculate M when t is five times the value calculated above.
Substituting in the model:
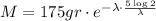
Simplifying (the value of λ cancels out):
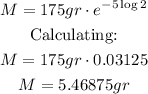
Rounding to the nearest gram, 5 grams of the radioactive isotope will be left after the required time.