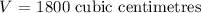SOLUTION:
Step 1:
In this question, we are given the following:
The volume of a gas V held at a constant temperature in a closed container varies inversely with its pressure P. If the volume of a gas is 800 cubic centimeters when the pressure is 450 millimeters of mercury (mm Hg), find the volume when the pressure is 200 mm Hg.
Step 2:
This is an application of Boyle's law which states that:
Boyle’s law is a gas law that states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant.
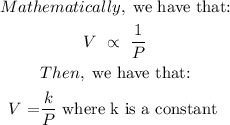
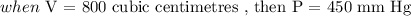
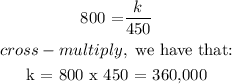
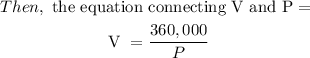
Next:
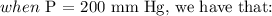
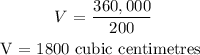
CONCLUSION:
The volume when the pressure is 200 mm Hg is: