Answer
The moles of water that will be produced = 0.343 mol
Step-by-step explanation
Given:
Mass of Ca(OH)2 = 29.0 g
Mass of HCl = 12.5 g
We know the reaction : Ca(OH)2 + 2HCI —> CaCI2 + 2H2O
Molar mass of Ca(OH)2 = 74.093 g/mol
Molar mass of HCl = 36.458 g/mol
Molar mass of H2O = 18.015 g/mol
Required: Moles of water that will be formed
Solution:
Use the stoichiometry to find the moles of water using both the reactants.
For Ca(OH)2:
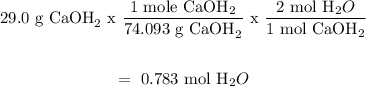
For HCl
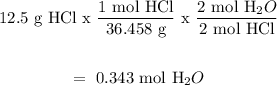
HCl will produce less moles of H2O, thus HCl is the limiting reactant/reagent and the moles of water that will be produced = 0.343 mol