Answer:
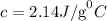
Explanations
The formula for calculating the amount of heat energy absorbed is given as:
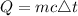
Given the following
• mass m = 50.0g
,
• Heat energy Q = 7490 Joules
,
• change in temperature = 80 -10= 70 degrees celsius
Substitute
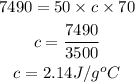
Hence the specific heat capacity of methanol is 2.14J/gC