Density relates the mass of a compound to its volume. We will first find the mass of the solution using the density and volume given. We have the following equation:
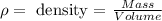
We clear the mass,
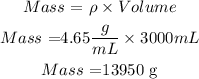
So, the mass of paint is 13950 g. Now the mass percentage of the lead in the paint will be calculated as follows:
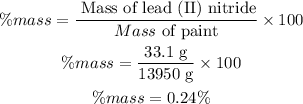
The mass percentage of lead in the paint is 0.24%.
ppm concentration means the quantity of solute in milligrams (mg) is in a liter (L) of a solution. So, to calculate ppm concentration we will divide the milligrams of lead (II) nitride between the liters of paint:

The ppm of lead in the paint is 11033ppm.