ANSWER
![undefined]()
Step-by-step explanation
Given that;
The mass of Al2(SO4)3 is 500 grams
The mass of Ca(OH)2 is 450 grams
The mass of CaSO4 is 596 grams
Follow the steps below to find the limiting reactant of the reaction
Step 1; Write the balanced equation of the reaction
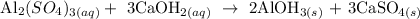
In the reaction above, 1 mole Al2(SO4)3 reacts with 3 moles Ca(OH)2 to give 2 moles Al(OH)3 and 3 moles CaSO4
Step 2; Determine the number of moles using the below formula
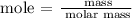
Recall, that the molar mass of Al2(SO4)3 is 342.15 g/mol and the molar mass of Ca(OH)2 is 74.093 g/mol
For Al2(SO4)3
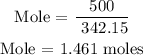
For Ca(OH)2
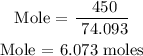
Step 3; Find the limiting reactant of the reaction
To find the limiting reactant of the reaction, divide the moles of the reactant by the co-efficient of the compound
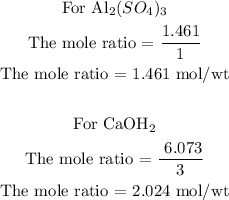
Since the limiting reactant of the reaction is the reactant with the lowest number of mol/wt, then Al2(SO4)3 is the limiting reactant
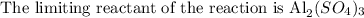
The excess reactant of the reaction is Ca(OH)2
Therefore, the no of moles of the excess reactant that is unreacted is
6.073 - 1.461 = 4.612 moles
Hence, the number of moles of the excess reactant that is unreacted is 4.621 moles