ANSWER
• I(toaster) = 15.08 A
,
• I(pan) = 11.17 A
,
• I(lamp) = 0.71 A
Step-by-step explanation
Given:
• The toaster's power, P(toaster) = 1810 W
,
• The frying pan's power, P(pan) = 1340 W
,
• The lamp's power, P(lamp) = 85 W
,
• The total current supported by the circuit, I = 15 A
,
• The voltage of the circuit, V = 120 V
Find:
• The current across each device
Power is the product of current and voltage,
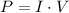
We know that all three devices are connected in parallel, which means that they all have the same voltage, 120 V.
Solving the equation above for I,
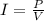
So, for each device,
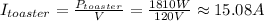
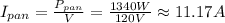
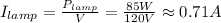
Hence, the current drawn by each device is:
• I(toaster) = 15.08 A
,
• I(pan) = 11.17 A
,
• I(lamp) = 0.71 A