Answer:
18.82L of oxygen gas are needed.
Step-by-step explanation:
1st) It is necessary to write and balance the chemical reaction:
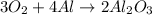
2nd) From the balanced reaction, we can see that 3 moles of oxygen gas (O2) react with 4 moles of aluminum (Al). To convert moles to grams, it is necessary to use the molar mass of oxygen (32g/mol) and aluminum (27g/mol):
- O2 conversion:
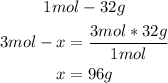
- Al conversion:
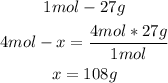
Now we can see that 96g of O2 react with 108g of Al.
3rd) We have to calculate the grams of O2 that will react with 30.25g of Al:
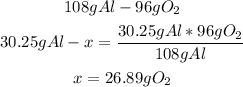
Using the molar mass of oxygen, we know that 26.89g represent 0.84 moles of O2.
4th) Finally, a mole of a gas at STP conditions occupies a volume of 22.4L. With this number and the moles of oxygen gas, we can calculate the liters:
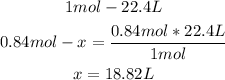
So, 18.82L of oxygen gas are needed.