Answer:
There are 2.2 moles of hydrogen.
Step-by-step explanation:
The given information from the exercise is:
- Volume (V): 48.6L
- Temperature (T): 0°C (273K)
- Pressure (P): 1atm
1st) It is important to convert the temperature unit from °C to Kelvin:
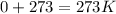
This first step is very important, because all the units must be at atm, L and Kelvin, before using the Ideal Gases Law formula.
2nd) Now to calculate the moles of hydrogen, we hae to replace the values of V, T and P in the Ideal Gases Law formula:
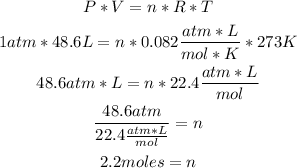
R: is the gases constant, in its value is 0.082atm.L/mol.K.
So, there are 2.2 moles of hydrogen.