ANSWER
A. 9600 kg
Step-by-step explanation
Given:
• The volume of the substance, V = 12m³
,
• The relative density of the substance, 0.8
,
• The density of water, 1000 kg/m³
Unknown:
• The mass of the substance, m
The relative density of a substance is defined as,
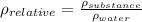
And the density of a substance is,
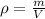
Let's solve the first formula for the density of the substance,
![\rho_(substance)=\rho_(relative)\cdot\rho_(water)=0.8\cdot1000\operatorname{kg}/m^3=800\operatorname{kg}/m^3]()
Then, solve the second formula for m,
![m=\rho\cdot V=800\operatorname{kg}/m^3\cdot12m^3=9600\operatorname{kg}]()
Hence, the mass of this substance is 9600 kg