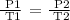ANSWER
The pressure of the gas is 281.25 mmHg
Step-by-step explanation
Given that;
The initial pressure of the gas is 75 mmHg
The intial temperature of the gas is 80K
The final temperature of the gas is 27 degrees Celcius
Follow the steps below to find the final pressure of the gas
Step 1; Convert the final temperature to degrees kelvin
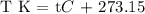
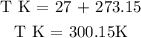
Step 2; Apply the Gay Lussac's law