The question requires us to calculate the amount of moles of water that we would have in 5.3x10^26 molecules of water.
To solve this problem, we'll need to consider the Avogadro Number: this number states the amount of particles (electrons, ions, atoms, molecules etc.) in one mole of a substance. The Avogadro Number corresponds to 6.022 x 10^23 particles per mol.
Considering the Avogadro number and the number of molecules provided by the question, we can write:
6.022 x 10^23 molecules of water -------------------- 1 mol of water
5.3 x 10^26 molecules of water ------------------------ x
Solving for x, we'll have:
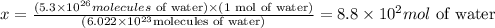
Therefore, there are 8.8 x 10^2 moles of water in 5.3 x 10^26 molecules of water.