Answer:
T = 101.96 degrees
Step-by-step explanation:
We are given the following
Number of moles = 0.650 mol
Volume = 1.00 L
Pressure = 20.00 atm
We are going to use the ideal gas low which is given by:
pV = nRT
Therefore:
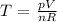
We know R = 0.08206 L.atm/Kmol
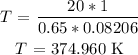
T(in degrees) = 374.96 - 273
= 101.96 degrees