Answer: the concentration of the solution after the dllution is 0.050 M
Step-by-step explanation:
The question requires us to determine the concentration of a solution after a dilution process.
The following information was provided by the question:
Concentration of initial solution = C1 = 0.30 M
Volume taken from initial solution = V1 = 25.0 mL
Volume of final solution = V2 = 150 mL
When a solution is diluted, the number of moles of the substance remains the same. Thus, we can say that:
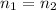
where (1) is the solution before dilution and (2) refers to the solution after dilution.
The number of moles of a substance can be obtained from the definition of molarity (or molar concentration), where the number of moles is divided by the volume of the solution:
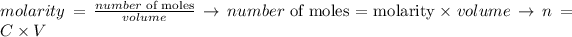
Thus, we can write that for a dillution:
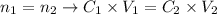
From the expression above we can determine the concentration of the final solution. Applying the values provided by the question to the expression above, we'll have:
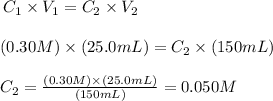
Therefore, the concentration of the solution after the dilution is 0.050 M.