Answer: ∆H total= -476.35kJ.
Explanations:
• We will follow Hess's Law of Constant Heat Summation:
(that states that if a reaction occurs in more than one route, then the the total enthalpy change for the reaction is the sum of all changes.)
For reaction :
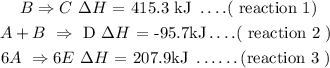
∆H total =( reaction 3 )/6 +reaction 2 - reaction 1
this can be expressed as :
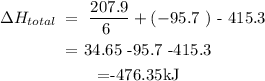
This means that ∆H total= -476.35kJ.