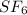The empirical formula corresponds to the simplest form of expressing a compound, it indicates the proportion of atoms in the molecule.
We have two elements S and F and they give us the mass resulting from the decomposition of the molecule. We can find the moles of each element using the atomic weight of each element as follows:
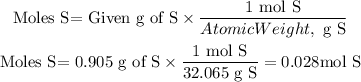
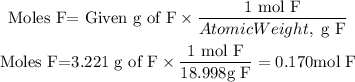
To find the ratio between the elements we divide the moles of each element by the smallest number of moles found, that is by 0.028 moles.

Therefore the empirical formula of the compound will be: